Comments
- No comments found
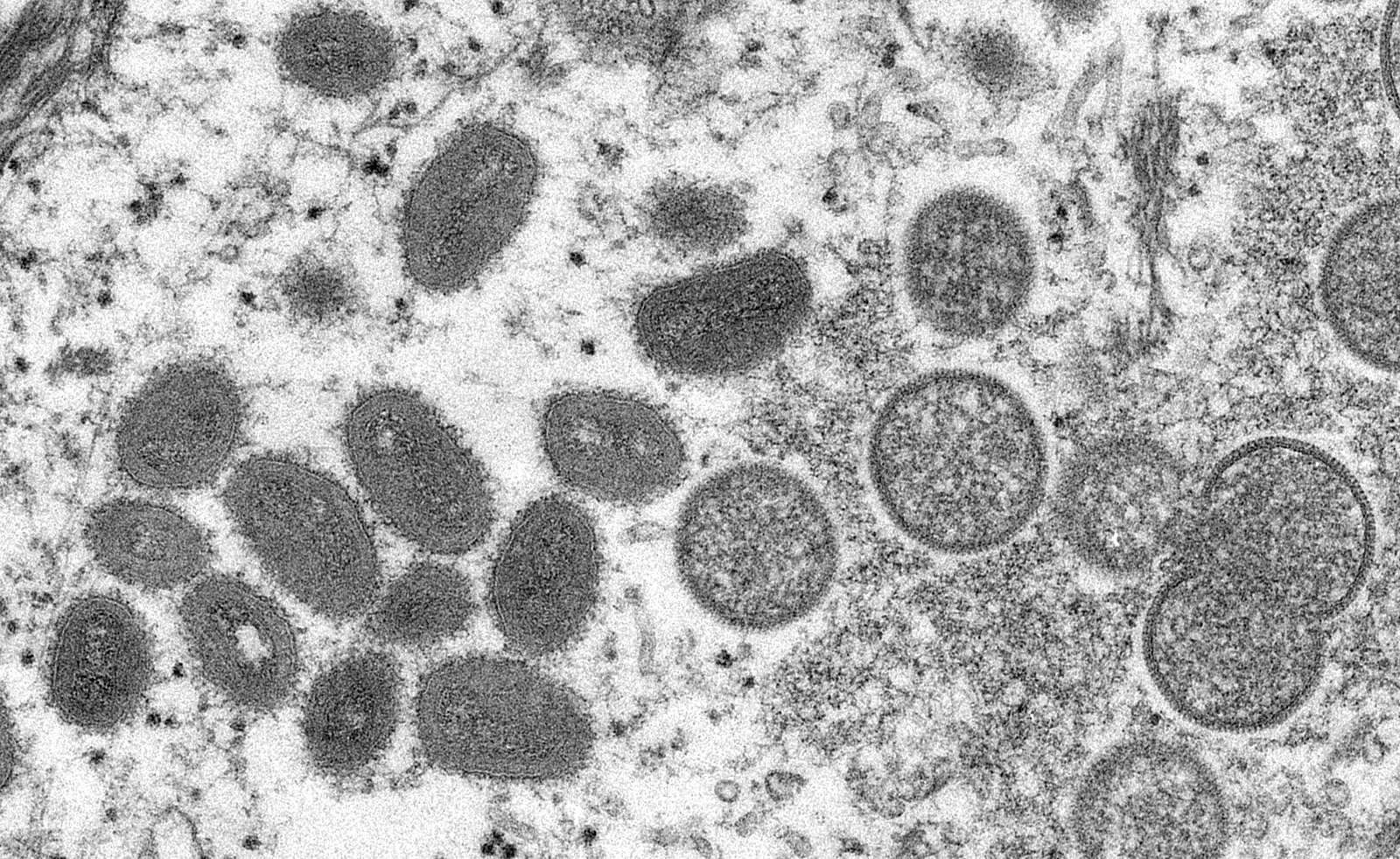
Monkeypox is an emerging viral epidemic lining out after the recent COVID-19 pandemic.
Vaccines won against the pandemic and vaccination for monkeypox is in effect across the United States.
However, it is necessary to explore new ways towards faster strikes against viral diseases before they take hold. The article below looks at possibilities with bubbles as an assistive therapeutic approach against viruses.
Viruses depend on hosts for replication. They mostly have a focus area adapting their damage for those cells, tissues, or organs. Some are respiratory pathogens. Others attack the gut, immune system, and so on.
The coronavirus replicates and multiplies in the blood, starting from the upper respiratory tract. This is similar also to other viruses emerging after contact then multiplying.
The normality of normal cells is strength for viral entry. When viruses emerge, they unbind and freewill around to continue their spread until they overwhelm the system.
How about a way to have cells unaligned, so the viral cycle is jolted, while enabling the immune system to rally and therapies take hold?
What this means is that rather than have cells in formation for the ease of viruses to slide in, there is a way to jounce cells, so that viruses are confused and struggle to find a host, as immune cells muster.
Bubbles are a possibility to nudge cells, in certain areas of the body, against certain viruses. The bubbles would contain two familiar gases to the bloodstream, oxygen and carbon dioxide. But they will be delivered in ways to avoid air embolism.
The purpose of both is to have polarity, so that whatever side cells choose or viruses flow, they could be tracked or targeted to be weakened or for expulsion.
If bubbles of carbon dioxide and oxygen are released to blood vessels in the respiratory area, it would create a space, induce diffusion and may absorb viruses.
The concentration, pressure, volume and intervals of will displace normal cells, including those already hosting viruses.
The reason this may work is because of hypoxia, hypocapnia and hypoxemia.
Happy hypoxia is a situation of COVID-19 where people are low on oxygen but appear just fine. Hypoxia was induced by the condition — necessitating supplemental oxygen for patients, helping many.
However, in the cases it is not enough against hypoxia, bubbles are released, with gases, one to seem friendly, and the other unfriendly, introducing confusion into the viral infected bloodstream.
O2 gas as bubbles — into arteries and capillaries.
Also, CO2 gas as bubbles — into the vein and venules.
Their form — in blood — will be similar to how bubbles diffuse in liquid where they don’t change state unless they hit a surface or get dissolved.
Computational Virology | Computational Hemodynamics
The first path is to have a computational blood flow model — to simulate ways this might work to assist meds, reduce pathogenic volume and for safe level releases against side effects.
Generative adversarial networks will be used to model parts of the lungs and blood vessels, with training data of viral-cellular interactions.
The objective is to seek out how it may help, at what stage, or with what viruses towards care. Cell culture experiments may then be tried subsequently, if it holds promise.
Leave your comments
Post comment as a guest